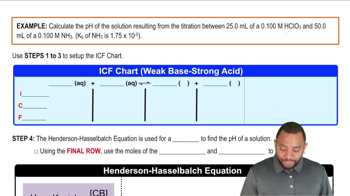
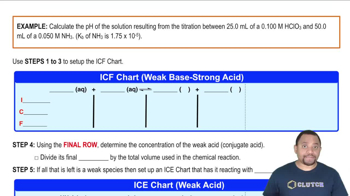
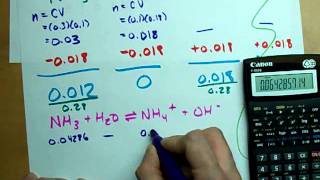18. Aqueous Equilibrium
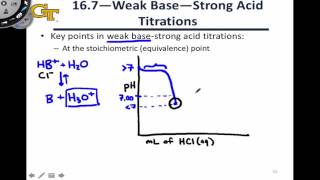
Titrations: Weak Base-Strong Acid

Get help from an AI Tutor
Ask a question to get started.
Problem 156
Textbook Question
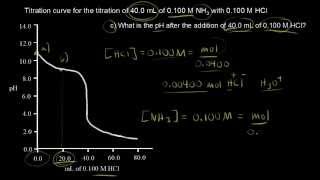
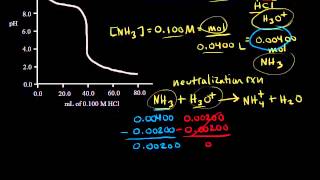
Textbook QuestionNeutralization reactions involving either a strong acid or a strong base go essentially to completion, and therefore we must take such neutralizations into account before calculating concentrations in mixtures of acids and bases. Consider a mixture of 3.28 g of Na3PO4 and 300.0 mL of 0.180 M HCl. Write balanced net ionic equations for the neutralization reactions and calculate the pH of the solution.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
28mPlay a video:
312
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos