10. Periodic Properties of the Elements
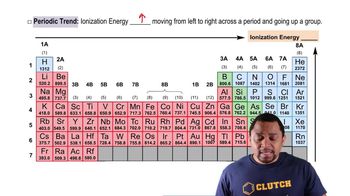
Periodic Trend: Ionization Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 43
Textbook Question
Textbook QuestionBased on their positions in the periodic table, predict which atom of the following pairs will have the smaller first ionization energy: (d) Pb, S
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
522
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos