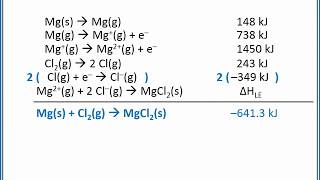11. Bonding & Molecular Structure
Born Haber Cycle

Get help from an AI Tutor
Ask a question to get started.
Problem 87
Textbook Question
Textbook QuestionThe estimated lattice energy for CsF21s2 is +2347 kJ/mol. Use the data given in Problem 6.86 to calculate an overall energy change in kilojoules per mole for the formation of CsF21s2 from its elements. Does the overall reaction absorb energy or release it? In light of your answer to Problem 6.86, which compound is more likely to form in the reaction of cesium with fluorine, CsF or CsF2?

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
827
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos