14. Solutions
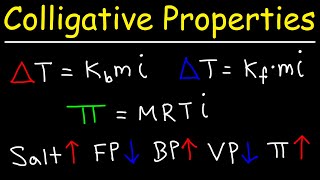
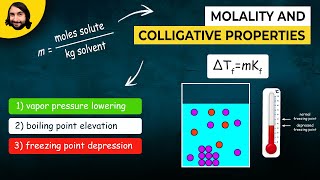
Freezing Point Depression

Get help from an AI Tutor
Ask a question to get started.
Problem 149b
Textbook Question
Textbook QuestionTreatment of 1.385 g of an unknown metal M with an excess of aqueous HCl evolved a gas that was found to have a volume of 382.6 mL at 20.0 °C and 755 mm Hg pressure. Heating the reaction mixture to evaporate the water and remaining HCl then gave a white crystalline compound, MClx. After dis- solving the compound in 25.0 g of water, the melting point of the resulting solution was - 3.53 °C. (c) What is the molality of particles (ions) in the solution of MClx?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
184
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 7 videos