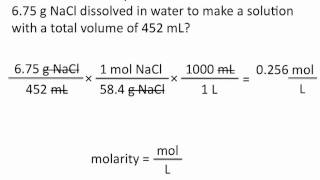
6. Chemical Quantities & Aqueous Reactions
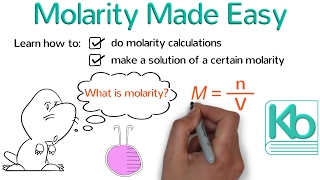
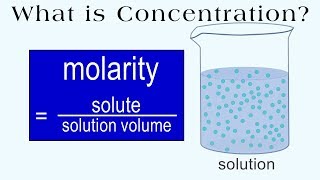
Molarity

Get help from an AI Tutor
Ask a question to get started.
Problem 64c
Textbook Question
Textbook QuestionA solution is prepared by dissolving 20.2 mL of methanol (CH3OH) in 100.0 mL of water at 25 °C. The final volume of the solution is 118 mL. The densities of methanol and water at this temperature are 0.782 g>mL and 1.00 g>mL, respectively. For this solution, calculate the concentration in each unit. a. molarity
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
820
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos