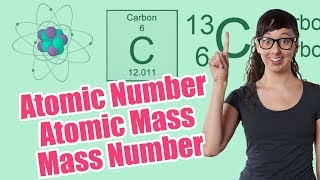2. Atoms & Elements
Atomic Mass

Get help from an AI Tutor
Ask a question to get started.
Problem 2a
Textbook Question
Textbook QuestionThe following diagram is a representation of 20 atoms of a fictitious element, which we will call nevadium (Nv). The red spheres are 293Nv, and the blue spheres are 295Nv. (b) If the mass of 293Nv is 293.15 u and that of 295Nv is 295.15 u, what is the atomic weight of Nv?

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
298
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 16 videos