2. Atoms & Elements
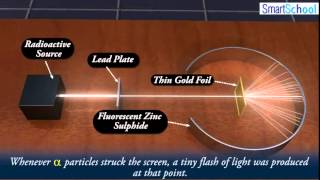
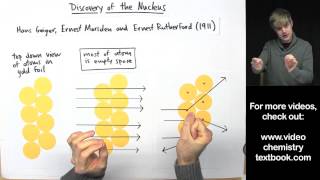
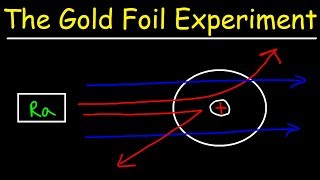
Rutherford Gold Foil Experiment

Get help from an AI Tutor
Ask a question to get started.
Problem 98a
Textbook Question
Textbook QuestionThere are two different isotopes of bromine atoms. Under normal conditions, elemental bromine consists of Br2 molecules, and the mass of a Br2 molecule is the sum of the masses of the two atoms in the molecule. The mass spectrum of Br2 consists of three peaks: Mass (u) Relative Size 157.836 0.2569 159.834 0.4999 161.832 0.2431 (e) Calculate the abundances of the two isotopes. Calculate the abundance of the heavier isotope.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
158
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos