3. Chemical Reactions
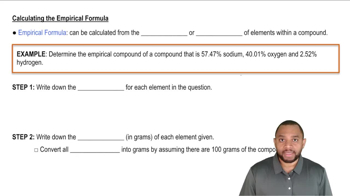
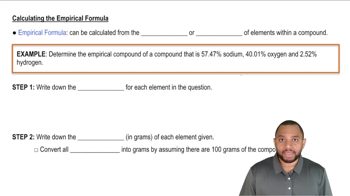
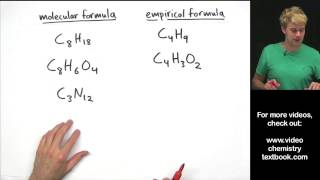
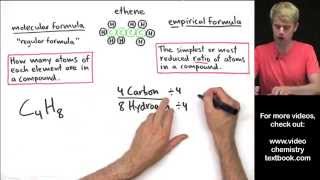
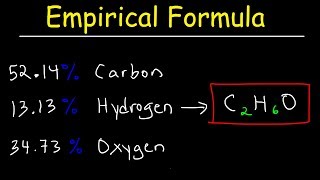
Empirical Formula

Get help from an AI Tutor
Ask a question to get started.
Problem 88d
Textbook Question
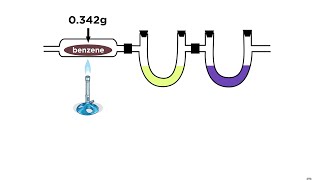
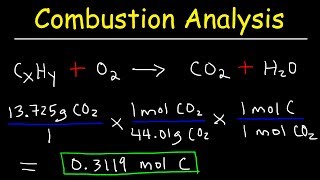
Textbook QuestionA chemist decomposes samples of several compounds; the masses of their constituent elements are listed. Calculate the empirical formula for each compound. c. 2.128 g Be, 7.557 g S, 15.107 g O
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
1694
views
2
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos