19. Chemical Thermodynamics
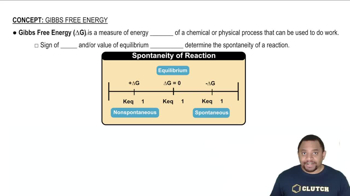
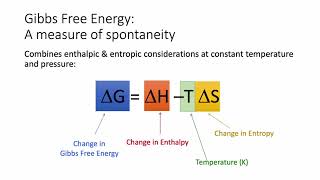
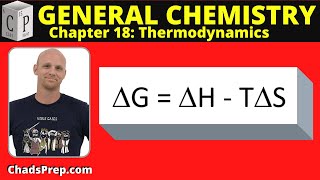
Gibbs Free Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 95a
Textbook Question
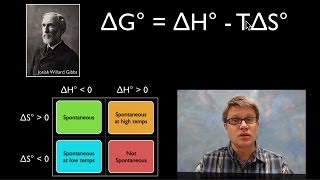
Textbook QuestionConsider the following three reactions: (i) Ti1s2 + 2 Cl21g2¡TiCl41g2 (ii) C2H61g2 + 7 Cl21g2 ¡2 CCl41g2 + 6 HCl1g2 (iii) BaO1s2 + CO21g2¡BaCO31s2 (c) For each of the reactions, predict the manner in which the change in free energy varies with an increase in temperature.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
228
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos