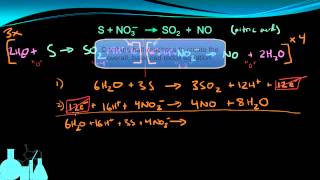6. Chemical Quantities & Aqueous Reactions
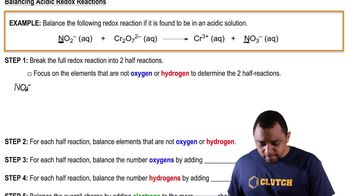
Balancing Redox Reactions: Acidic Solutions

Get help from an AI Tutor
Ask a question to get started.
Problem 154c
Textbook Question
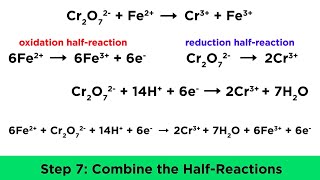
Textbook QuestionTo 100.0 mL of a solution that contains 0.120 M Cr(NO3)2 and 0.500 M HNO3 is added to 20.0 mL of 0.250 M K2Cr2O7. The dichromate and chromium(II) ions react to give chromium(III) ions. (a) Write a balanced net ionic equation for the reaction.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
308
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos