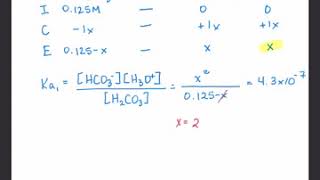17. Acid and Base Equilibrium
Diprotic Acids and Bases

Get help from an AI Tutor
Ask a question to get started.
Problem 119
Textbook Question
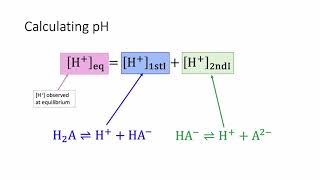
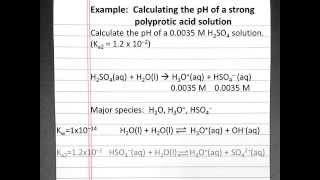
Textbook QuestionAtmospheric CO2 levels have risen by nearly 20% over the past 40 years from 320 ppm to 400 ppm. (a) Given that the average pH of clean, unpolluted rain today is 5.4, determine the pH of unpolluted rain 40 years ago. Assume that carbonic acid 1H2CO32 formed by the reaction of CO2 and water is the only factor influencing pH. CO21g2 + H2O1l2 Δ H2CO31aq2
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
13mPlay a video:
262
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos