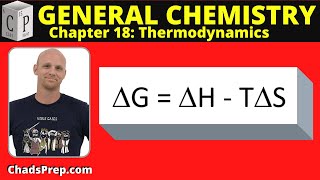
19. Chemical Thermodynamics
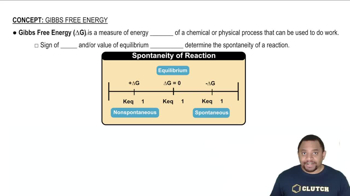
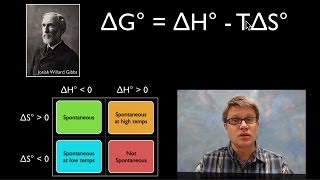
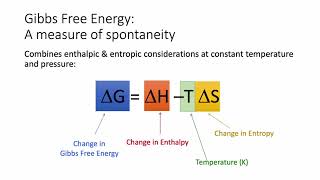
Gibbs Free Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 136
Textbook Question
Textbook QuestionA mixture of 14.0 g of N2 and 3.024 g of H2 in a 5.00 L container is heated to 400 °C. Use the data in Appendix B to calculate the molar concentrations of N2, H2, and NH3 at equilibrium. Assume that ∆H° and ∆S° are independent of temperature, and remember that the standard state of a gas is defined in terms of pressure.

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
11mPlay a video:
372
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos