14. Solutions
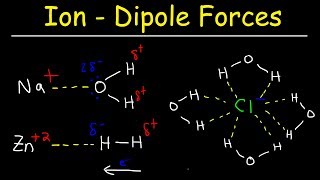
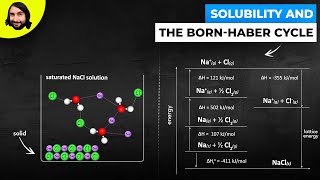
Solutions: Solubility and Intermolecular Forces

Get help from an AI Tutor
Ask a question to get started.
Problem 114
Textbook Question
Textbook QuestionCompounds like sodium stearate, called 'surfactants' in general, can form structures known as micelles in water, once the solution concentration reaches the value known as the critical micelle concentration (cmc). Micelles contain dozens to hundreds of molecules. The cmc depends on the substance, the solvent, and the temperature. (a) The turbidity (the amount of light scattering) of solutions increases dramatically at the cmc. Suggest an explanation. .
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
486
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 16 videos