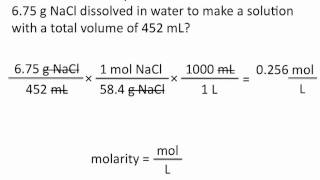
6. Chemical Quantities & Aqueous Reactions
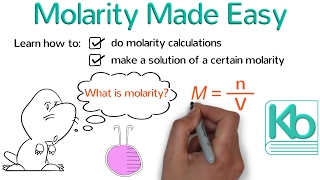
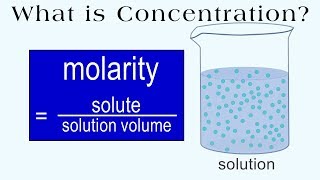
Molarity

Get help from an AI Tutor
Ask a question to get started.
Problem 98
Textbook Question
Textbook QuestionBronze is a solid solution of Cu(s) and Sn(s); solutions of metals like this that are solids are called alloys. There is a range of compositions over which the solution is considered a bronze. Bronzes are stronger and harder than either copper or tin alone. (b) Based on part (a), calculate the concentration of the solute metal in the alloy in units of molarity, assuming a density of 7.9 g/cm3.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1618
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos