7. Gases
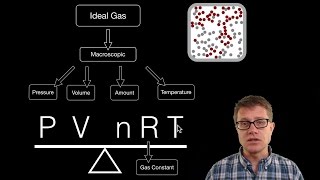
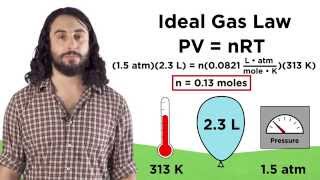
The Ideal Gas Law

Get help from an AI Tutor
Ask a question to get started.
Problem 74
Textbook Question
Textbook QuestionThe reaction of sodium peroxide 1Na2O22 with CO2 is used in space vehicles to remove CO2 from the air and generate O2 for breathing: 2 Na2O21s2 + 2 CO21g2¡2 Na2CO31s2 + O21g2 (a) Assuming that air is breathed at an average rate of 4.50 L/min (25 °C; 735 mm Hg) and that the concentration of CO2 in expelled air is 3.4% by volume, how many grams of CO2 are produced in 24 h?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
700
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos