10. Periodic Properties of the Elements
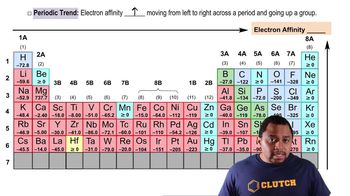
Periodic Trend: Electron Affinity

Get help from an AI Tutor
Ask a question to get started.
Problem 99
Textbook Question
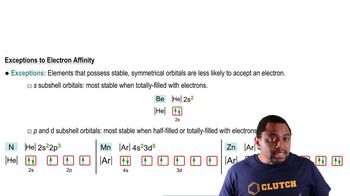
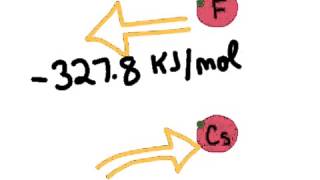
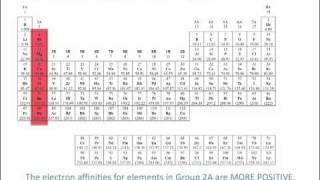
Textbook QuestionHydrogen is an unusual element because it behaves in some ways like the alkali metal elements and in other ways like nonmetals. Its properties can be explained in part by its electron configuration and by the values for its ionization energy and electron affinity. (a) Explain why the electron affinity of hydrogen is much closer to the values for the alkali elements than for the halogens.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
1029
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos