8. Thermochemistry
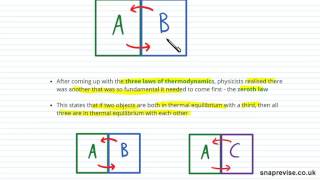
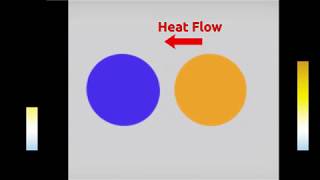
Thermal Equilibrium

Get help from an AI Tutor
Ask a question to get started.
Problem 103
Textbook Question
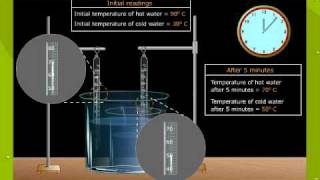
Textbook QuestionA 25.5-g aluminum block is warmed to 65.4 °C and plunged into an insulated beaker containing 55.2 g water initially at 22.2 °C. The aluminum and the water are allowed to come to thermal equilibrium. Assuming that no heat is lost, what is the final temperature of the water and aluminum?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
2046
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos