11. Bonding & Molecular Structure
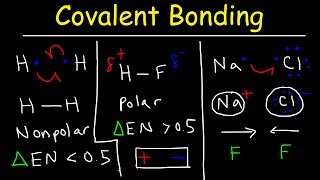
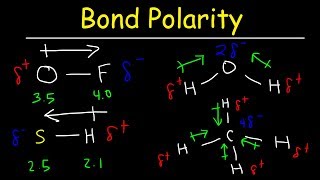
Dipole Moment

Get help from an AI Tutor
Ask a question to get started.
Problem 86
Textbook Question
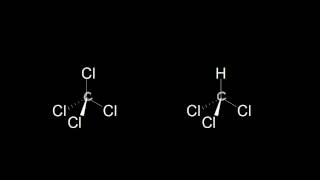
Textbook QuestionThe substance chlorine monoxide, ClO(g), is important in atmospheric processes that lead to depletion of the ozone layer. The ClO molecule has an experimental dipole moment of 1.24 D, and the Cl¬O bond length is 160 pm. (b) Based on the electronegativities of the elements, which atom would you expect to have a partial negative charge in the ClO molecule?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
754
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos