8. Thermochemistry
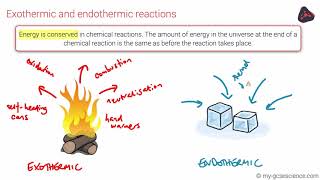
Endothermic & Exothermic Reactions

Get help from an AI Tutor
Ask a question to get started.
Problem 4
Textbook Question
Textbook QuestionThe contents of the closed box in each of the following illustrations represent a system, and the arrows show the changes to the system during some process. The lengths of the arrows represent the relative magnitudes of q and w. (a) Which of these processes is endothermic?

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
554
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos