7. Gases
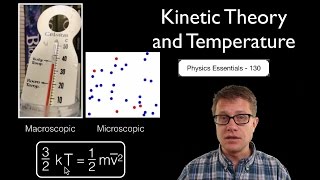
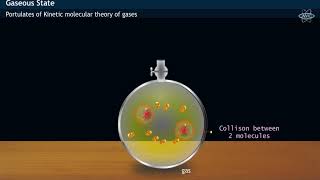
Kinetic Molecular Theory

Get help from an AI Tutor
Ask a question to get started.
Problem 1
Textbook Question
Textbook QuestionAt 273 K and 1 atm pressure, 1 mol of an ideal gas occupies 22.4 L. (Section 10.4) (c) In which parts of the atmosphere would you expect gases to behave most ideally (ignoring any photochemical reactions)? [Section 18.1]
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
755
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos