19. Chemical Thermodynamics
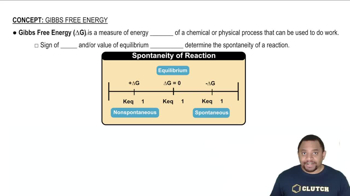
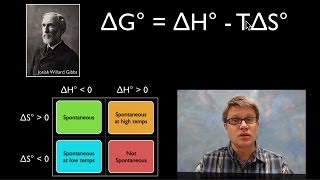
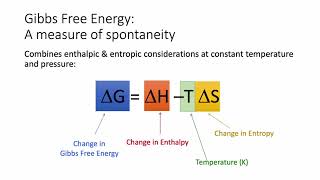
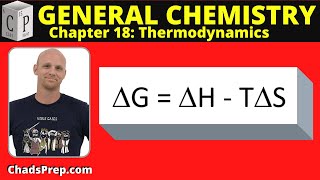
Gibbs Free Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 83c
Textbook Question
Textbook QuestionThe value of Ka for nitrous acid 1HNO22 at 25 °C is given in Appendix D. (d) What is the value of ΔG when 3H+4 = 5.0 * 10-2 M, 3NO2 -4 = 6.0 * 10-4 M, and 3HNO24 = 0.20 M?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
220
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos