14. Solutions
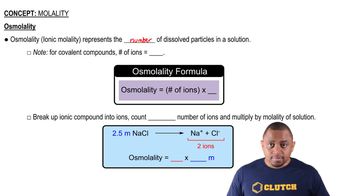
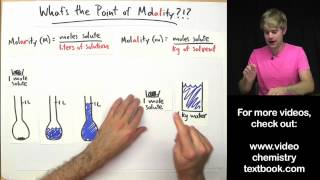
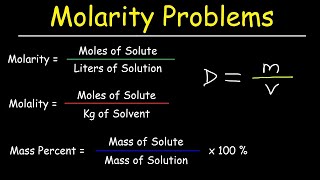
Molality

Get help from an AI Tutor
Ask a question to get started.
Problem 106
Textbook Question
Textbook QuestionFluorocarbons (compounds that contain both carbon and fluorine) were, until recently, used as refrigerants. The compounds listed in the following table are all gases at 25 °C, and their solubilities in water at 25 °C and 1 atm fluorocarbon pressure are given as mass percentages. (a) For each fluorocarbon, calculate the molality of a saturated solution. Fluorocarbon Solubility (mass %) CF4 0.0015 CClF3 0.009 CCl2F2 0.028 CHClF2 0.30
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
293
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos