6. Chemical Quantities & Aqueous Reactions
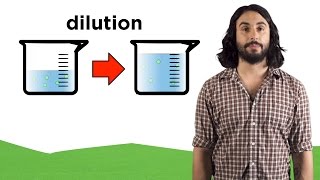
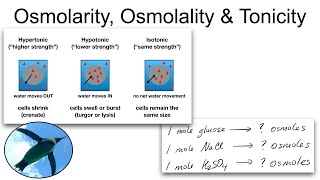
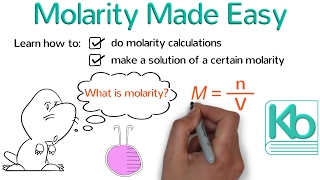
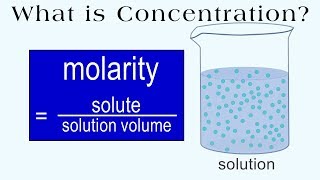
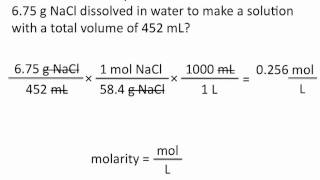
Molarity

Get help from an AI Tutor
Ask a question to get started.
Problem 64a
Textbook Question
Textbook QuestionA person suffering from hyponatremia has a sodium ion concentration in the blood of 0.118 M and a total blood volume of 4.6 L. What mass of sodium chloride would need to be added to the blood to bring the sodium ion concentration up to 0.138 M, assuming no change in blood volume?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
979
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos