13. Liquids, Solids & Intermolecular Forces
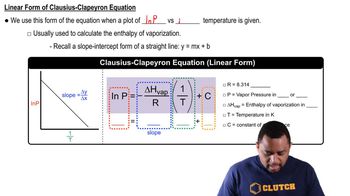
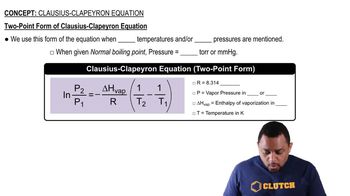
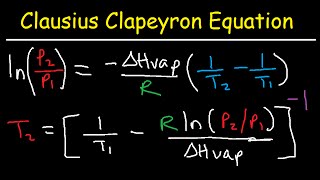
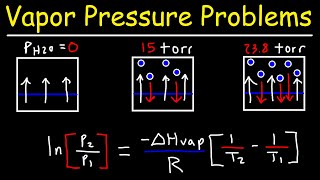
Clausius-Clapeyron Equation

Get help from an AI Tutor
Ask a question to get started.
Problem 61
Textbook Question
Textbook QuestionThis table displays the vapor pressure of ammonia at several different temperatures. Use the data to determine the heat of vaporization and normal boiling point of ammonia. Temperature (K) Pressure (torr) 200 65.3 210 134.3 220 255.7 230 456.0 235 597.0
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
1969
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos