2. Atoms & Elements
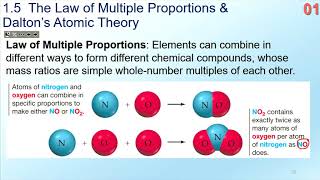
Law of Multiple Proportions

Get help from an AI Tutor
Ask a question to get started.
Problem 166
Textbook Question
Textbook QuestionAmmonia (NH3) and hydrazine (N2H4) are both compounds of nitrogen and hydrogen. Based on the law of multiple pro-portions, how many grams of hydrogen would you expect 2.34 g of nitrogen to combine with to yield ammonia? To yield hydrazine?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
892
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos