15. Chemical Kinetics
Reaction Mechanism

Get help from an AI Tutor
Ask a question to get started.
Problem 114a
Textbook Question
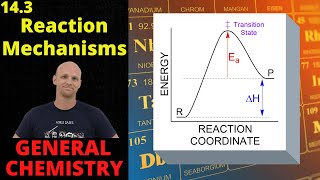
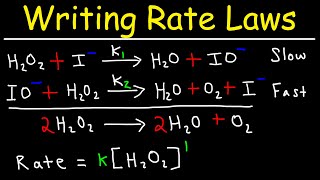
Textbook QuestionA proposed mechanism for the oxidation of nitric oxide to nitrogen dioxide was described in Problem 14.29. Another possible mechanism for this reaction is 2 NO1g2 k1 k-1 N2O21g2 Faster, reversible N2O21g2 + O21g2 ¡ k2 2NO21g2 Slower, rate-determining (b) Show that this mechanism is consistent with the experimental rate law, Rate = k3NO423O24.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
511
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos