15. Chemical Kinetics
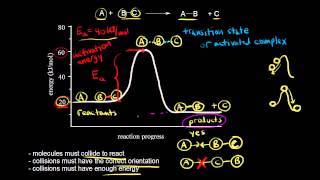
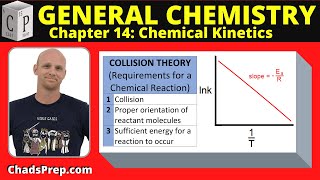
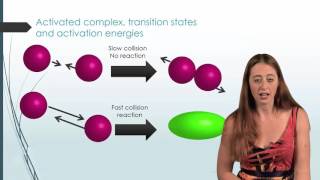
Collision Theory

Get help from an AI Tutor
Ask a question to get started.
Problem 120
Textbook Question
Textbook QuestionThe gas-phase reaction of NO with F2 to form NOF and F has an activation energy of Ea = 6.3 kJ>mol. and a frequency factor of A = 6.0 * 108 M-1 s-1. The reaction is believed to be bimolecular: NO1g2 + F21g2 ¡ NOF1g2 + F1g2 (e) Suggest a reason for the low activation energy for the reaction.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
597
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos