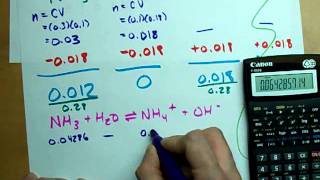18. Aqueous Equilibrium
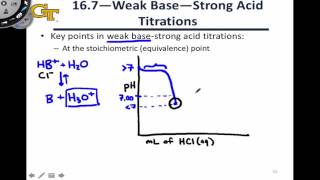
Titrations: Weak Base-Strong Acid

Get help from an AI Tutor
Ask a question to get started.
Problem 3
Textbook Question
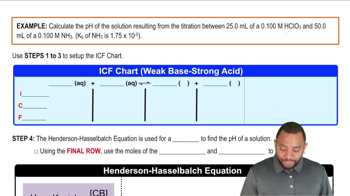
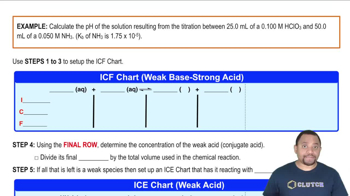
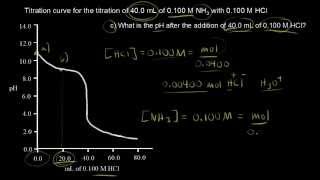
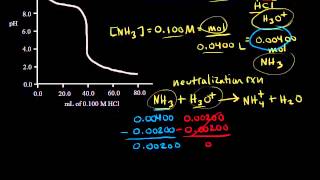
Textbook QuestionA 1.00 L buffer solution is 0.250 M in HF and 0.250 M in NaF. Calculate the pH of the solution after the addition of 100.0 mL of 1.00 M HCl. (Ka = 3.5 x 10^-4) (a) 4.11 (b) 3.82 (c) 3.46 (d) 3.09
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
8mPlay a video:
1587
views
2
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos