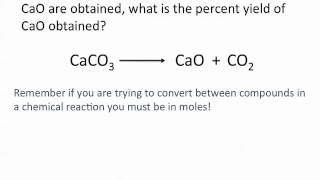
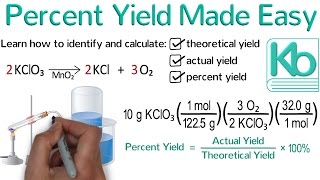
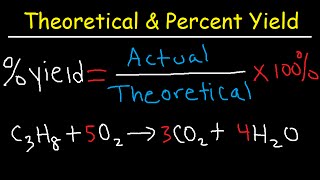
3. Chemical Reactions
Percent Yield

Get help from an AI Tutor
Ask a question to get started.
Problem 86
Textbook Question
Textbook QuestionWhen hydrogen sulfide gas is bubbled into a solution of sodium hydroxide, the reaction forms sodium sulfide and water. How many grams of sodium sulfide are formed if 1.25 g of hydrogen sulfide is bubbled into a solution containing 2.00 g of sodium hydroxide, assuming that the sodium sulfide is made in 92.0% yield?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
2170
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos