8. Thermochemistry
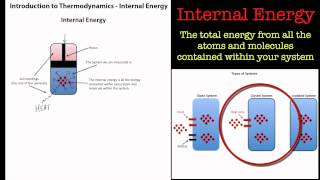
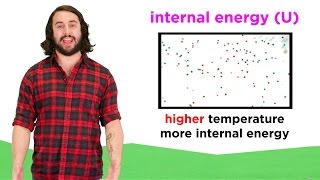
Internal Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 54c
Textbook Question
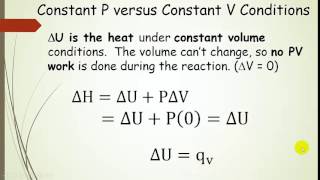
Textbook QuestionA gas is compressed from an initial volume of 5.55 L to a final volume of 1.22 L by an external pressure of 1.00 atm. During the compression the gas releases 124 J of heat. What is the change in internal energy of the gas?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
2482
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos