19. Chemical Thermodynamics
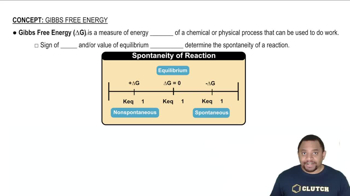
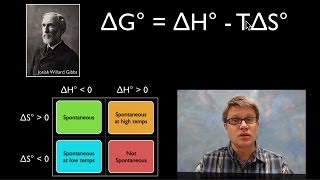
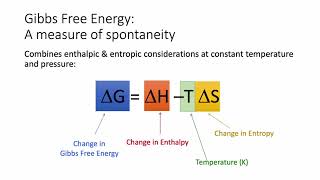
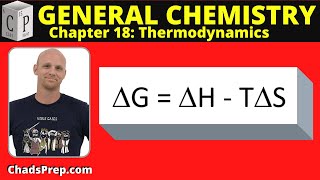
Gibbs Free Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 62c
Textbook Question
Textbook QuestionFor each reaction, calculate ΔHrxn ° , ΔSrxn ° , and ΔGrxn ° at 25 °C and state whether or not the reaction is spontaneous. If the reaction is not spontaneous, would a change in temperature make it spontaneous? If so, should the temperature be raised or lowered from 25 °C? d. 2 KClO3(s) ¡ 2 KCl(s) + 3 O2( g)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
9mPlay a video:
298
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos