19. Chemical Thermodynamics
Entropy

Get help from an AI Tutor
Ask a question to get started.
Problem 37
Textbook Question
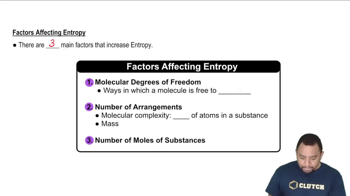
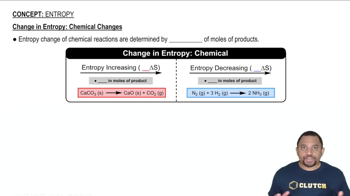
Textbook QuestionWithout doing any calculations, determine the signs of ΔSsys and ΔS surr for each chemical reaction. In addition, predict under what temperatures (all temperatures, low temperatures, or high temperatures), if any, the reaction is spontaneous. a. C3H8( g) + 5 O2( g) ¡ 3 CO2( g) + 4 H2O( g) ΔH° rxn = -2044 kJ
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
2372
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 16 videos