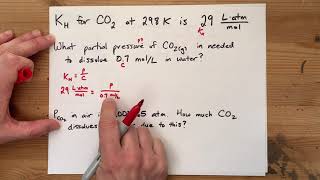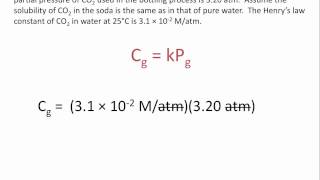14. Solutions
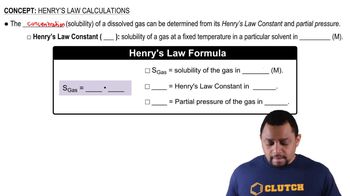
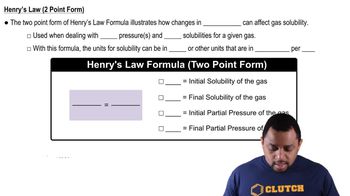
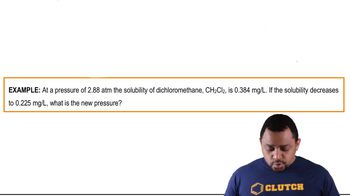
Henry's Law Calculations

Get help from an AI Tutor
Ask a question to get started.
Problem 85
Textbook Question
Textbook QuestionAt an altitude of 10,000 ft, the partial pressure of oxygen in the lungs is about 68 mm Hg. What is the concentration in mg/L of dissolved O2 in blood (or water) at this partial pres- sure and a normal body temperature of 37 °C? The solubil- ity of O2 in water at 37 °C and 1 atm partial pressure is 1.93 * 10-3 mol>L.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
581
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos