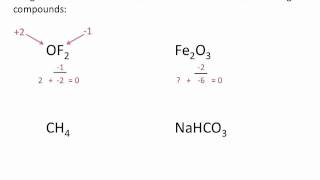6. Chemical Quantities & Aqueous Reactions
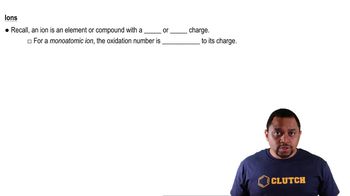
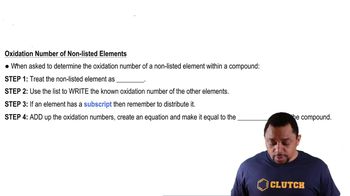
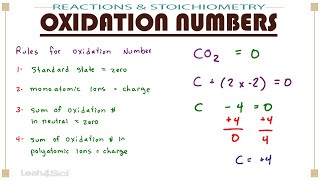
Calculate Oxidation Numbers

Get help from an AI Tutor
Ask a question to get started.
Problem 87a
Textbook Question
Textbook QuestionIron corrodes to produce rust, Fe2O3, but other corrosion products that can form are Fe(O)(OH), iron oxyhydroxide, and magnetite, Fe3O4. (a) What is the oxidation number of Fe in iron oxyhydroxide, assuming oxygen's oxidation number is - 2?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
969
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 16 videos