19. Chemical Thermodynamics
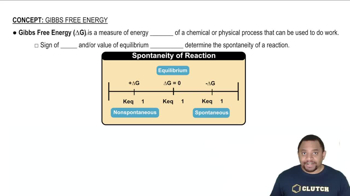
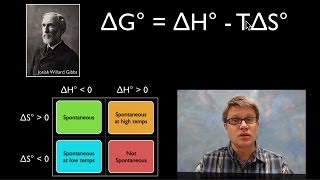
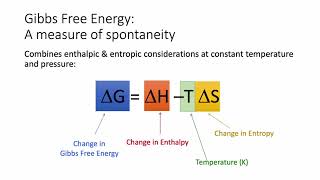
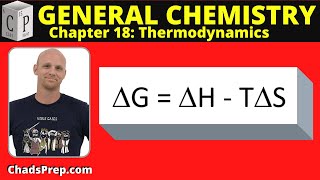
Gibbs Free Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 89a
Textbook Question
Textbook QuestionConsider this reaction occurring at 298 K: N2O( g) + NO2( g) ∆ 3 NO( g) c. Can the reaction be made more spontaneous by an increase or decrease in temperature? If so, what temperature is required to make the reaction spontaneous under standard conditions?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
8mPlay a video:
349
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos