7. Gases
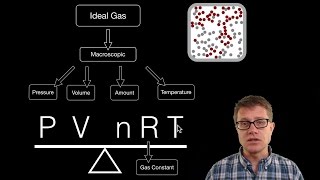
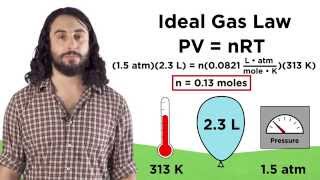
The Ideal Gas Law

Get help from an AI Tutor
Ask a question to get started.
Problem 45a
Textbook Question
Textbook QuestionIn an experiment reported in the scientific literature, male cockroaches were made to run at different speeds on a miniature treadmill while their oxygen consumption was measured. In 30 minutes the average cockroach (running at 0.08 km/h) consumed 1.0 mL of O2 at 101.33 kPa pressure and 20 °C per gram of insect mass. (a) How many moles of O2 would be consumed in 1 day by a 6.3-g cockroach moving at this speed?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
602
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos