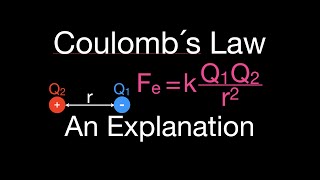11. Bonding & Molecular Structure
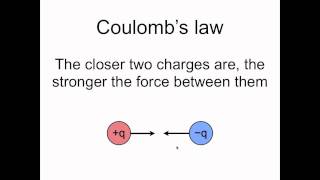
Coulomb's Law

Get help from an AI Tutor
Ask a question to get started.
Problem 111
Textbook Question
Textbook QuestionUse Coulomb's law to calculate the ionization energy in kJ>mol of an atom composed of a proton and an electron separated by 100.00 pm. What wavelength of light has sufficient energy to ionize the atom?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
15mPlay a video:
2366
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos