18. Aqueous Equilibrium
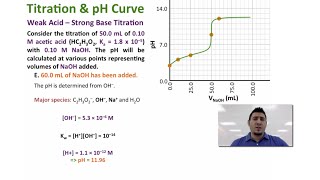
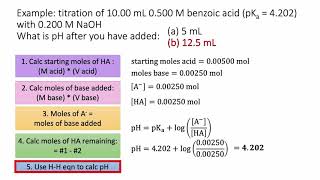
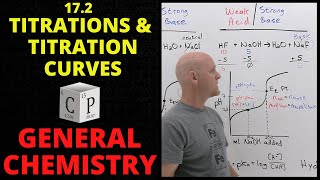
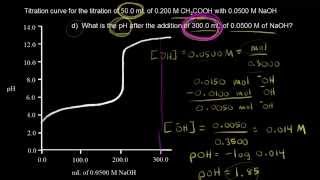
Titrations: Weak Acid-Strong Base

Get help from an AI Tutor
Ask a question to get started.
Problem 72e
Textbook Question
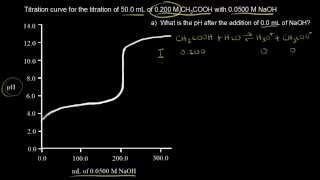
Textbook QuestionA 30.0-mL sample of 0.165 M propanoic acid is titrated with 0.300 M KOH. Calculate the pH at each volume of added base: equivalence point.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
10mPlay a video:
2238
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos