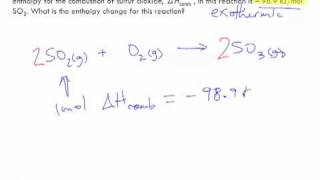8. Thermochemistry
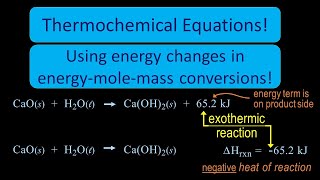
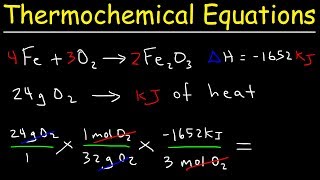
Thermochemical Equations

Get help from an AI Tutor
Ask a question to get started.
Problem 97
Textbook Question
Textbook QuestionAt the end of 2012, global population was about 7.0 billion people. What mass of glucose in kg would be needed to provide 1500 Cal/person/day of nourishment to the global population for one year? Assume that glucose is metabolized entirely to CO21g2 and H2O1l2 according to the following thermochemical equation: C6H12O61s2 + 6 O21g2¡6 CO21g2 + 6 H2O1l2 H = -2803 kJ
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
620
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos