7. Gases
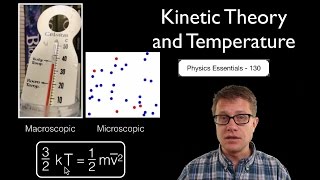
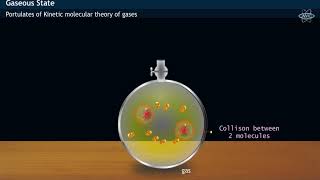
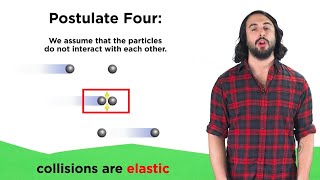
Kinetic Molecular Theory

Get help from an AI Tutor
Ask a question to get started.
Problem 108a
Textbook Question
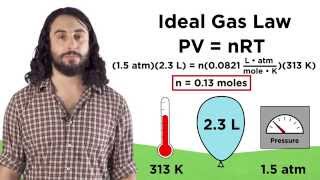
Textbook QuestionTwo 112-L tanks are filled with gas at 330 K. One contains 5.00 mol of Kr, and the other contains 5.00 mol of O2. Considering the assumptions of kinetic–molecular theory, rank the gases from low to high for each of the following properties. (d) Pressure
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
377
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos