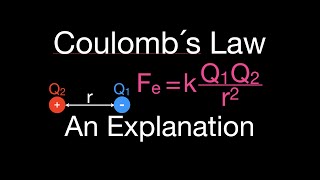11. Bonding & Molecular Structure
Coulomb's Law

Get help from an AI Tutor
Ask a question to get started.
Problem 15
Textbook Question
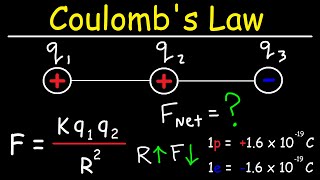
Textbook Question(a) The electrostatic force (not energy) of attraction between two oppositely charged objects is given by the equation F = k1Q1Q2>d22where k = 8.99 * 109 N@m2>C2 , Q1 and Q2 are the charges of the two objects in Coulombs, and d is the distance separating the two objects in meters. What is the electrostatic force of attraction (in Newtons) between an electron and a proton that are separated by 0.23 nm?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1255
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos