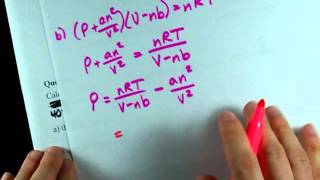7. Gases
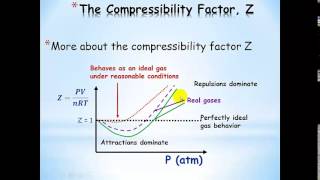
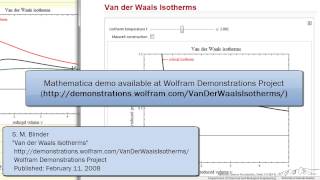
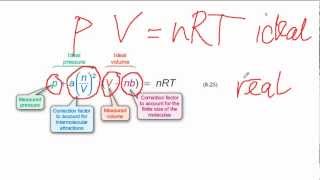
Van der Waals Equation

Get help from an AI Tutor
Ask a question to get started.
Problem 148
Textbook Question
Textbook QuestionThe Rankine temperature scale used in engineering is to the Fahrenheit scale as the Kelvin scale is to the Celsius scale. That is, 1 Rankine degree is the same size as 1 Fahrenheit degree, and 0 °R = absolute zero. (b) What is the value of the gas constant R on the Rankine scale in 1L ~ atm2>1°R ~ mol2? (c) Use the van der Waals equation to determine the pressure inside a 400.0-mL vessel that contains 2.50 mol of CH4 at a temperature of 525 °R. For CH4, a = 2.253 1L2 ~ atm2>mol2 and b = 0.04278 L>mol.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
9mPlay a video:
402
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos