8. Thermochemistry
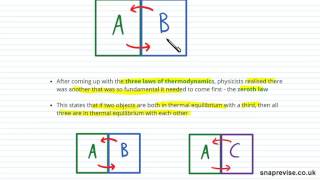
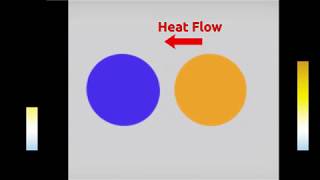
Thermal Equilibrium

Get help from an AI Tutor
Ask a question to get started.
Problem 107
Textbook Question
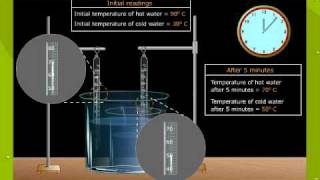
Textbook QuestionAn ice cube with a mass of 20 g at -20 °C (typical freezer temperature) is dropped into a cup that holds 500 mL of hot water, initially at 83 °C. What is the final temperature in the cup? The density of liquid water is 1.00 g>mL; the specific heat capacity of ice is 2.03 J>g@C; the specific heat capacity of liquid water is 4.184 J>g@C; the enthalpy of fusion of water is 6.01 kJ>mol.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
1883
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos