7. Gases
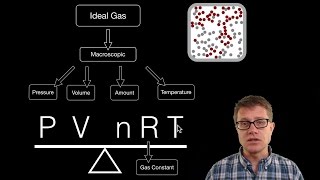
The Ideal Gas Law

Get help from an AI Tutor
Ask a question to get started.
Problem 41
Textbook Question
Textbook QuestionA 50.0 g sample of solid CO2 (dry ice) is added at -100 °C to an evacuated (all of the gas removed) container with a volume of 5.0 L. If the container is sealed and then allowed to warm to room temperature 125 °C2 so that the entire solid CO2 is converted to a gas, what is the pressure inside the container?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
1127
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos