8. Thermochemistry
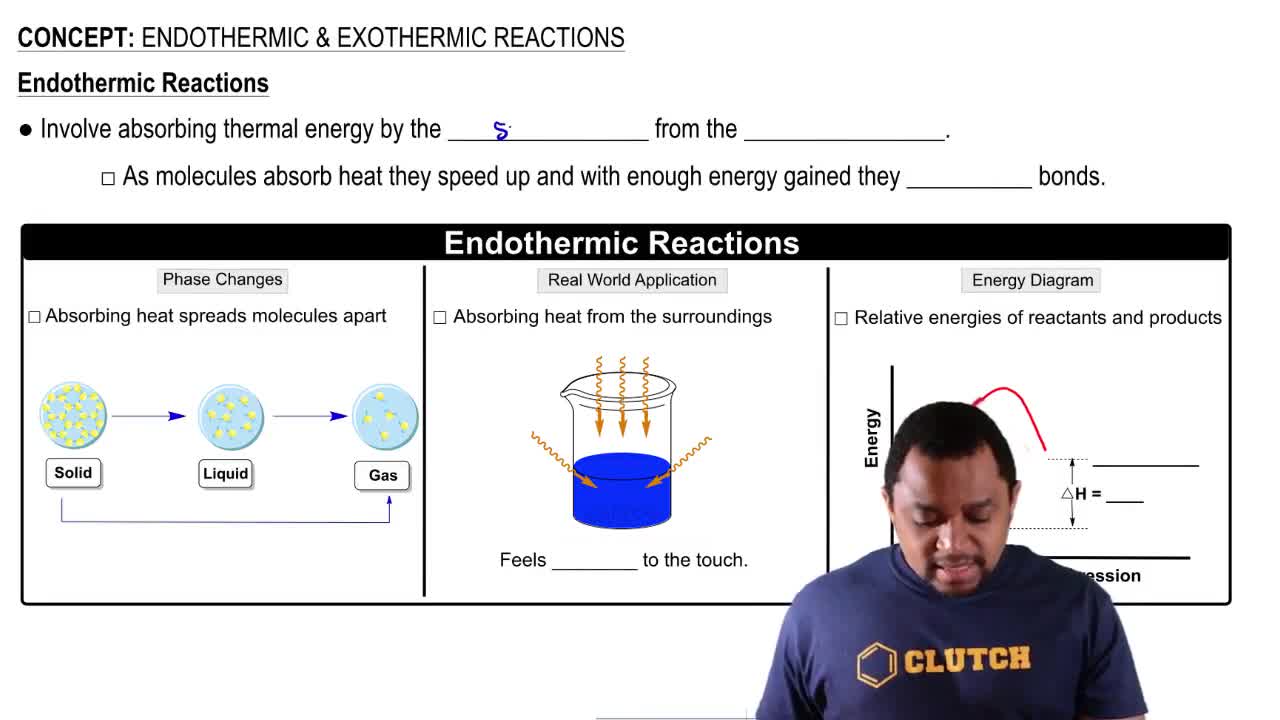
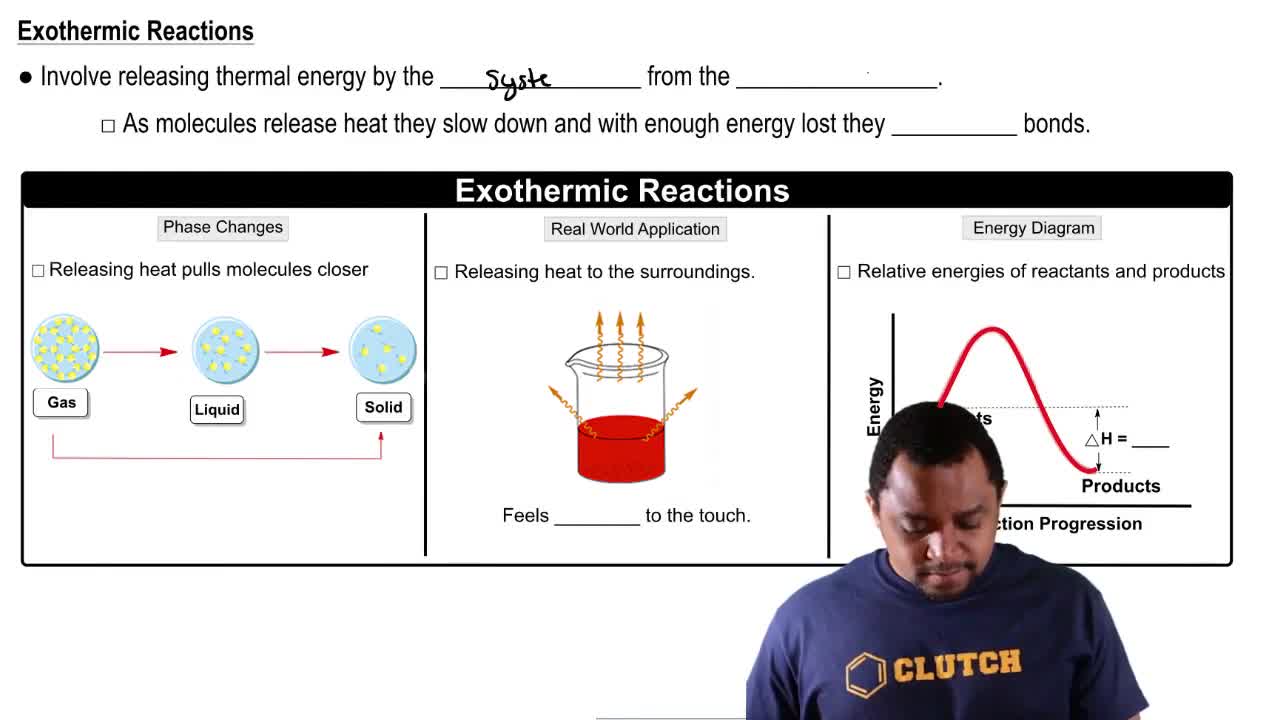
Endothermic & Exothermic Reactions

Get help from an AI Tutor
Ask a question to get started.
Problem 115
Textbook Question
Textbook QuestionThe thermite reaction, Fe2O3 + Al S Al2O3 + Fe produces so much heat that the Fe product melts. This reaction is used industrially to weld metal parts under water, where a torch cannot be employed. It is also a favorite chemical demonstration in the lecture hall (on a small scale). (d) If you performed the reverse reaction— aluminum oxide plus iron makes iron oxide plus aluminum—would that reaction have heat as a reactant or a product?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
1172
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos