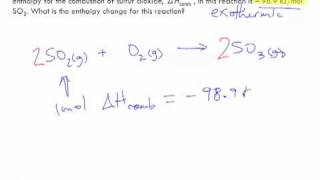8. Thermochemistry
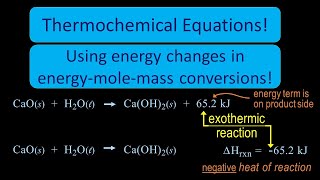
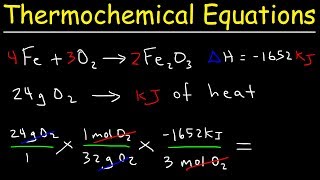
Thermochemical Equations

Get help from an AI Tutor
Ask a question to get started.
Problem 112
Textbook Question
Textbook QuestionWhen 10.00 g of phosphorus is burned in O2( g) to form P4O10(s), enough heat is generated to raise the temperature of 2950 g of water from 18.0 °C to 38.0 °C. Calculate the enthalpy of formation of P4O10(s) under these conditions.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1853
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos