10. Periodic Properties of the Elements
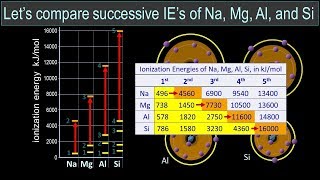
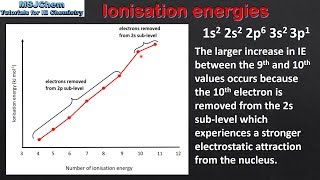
Periodic Trend: Successive Ionization Energies

Get help from an AI Tutor
Ask a question to get started.
Problem 7
Textbook Question
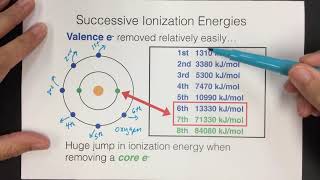
Textbook QuestionThe successive ionization energies for a second-period element are given. What is the identity of the element? (LO 6.8) Ea1 = 1402 kJ/mol Ea2 = 2856 kJ/mol Ea3 = 4578 kJ/mol Ea4 = 7475 kJ/mol Ea5 = 9445 kJ/mol Ea6 = 53,266 kJ/mol Ea7 = 64,630 kJ/mol (a) Be (b) C (c) N (d) F
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
449
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos