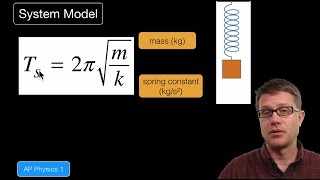
8. Thermochemistry
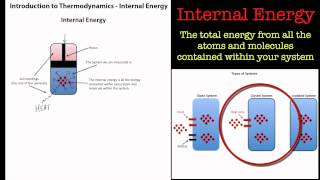
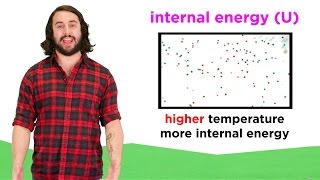
Internal Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 102
Textbook Question
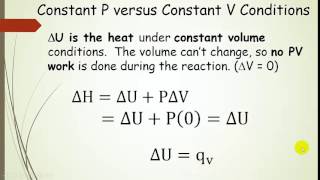
Textbook QuestionA sample of gas is contained in a cylinder-and-piston arrangement. There is an external pressure of 100 kPa. The gas undergoes the change in state shown in the drawing. (b) Now assume that the cylinder and piston are made up of a thermal conductor such as a metal. During the state change, the cylinder gets colder to the touch. What is the sign of q for the state change in this case? Describe the difference in the state of the system at the end of the process in the two cases. What can you say about the relative values of E?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
369
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos