10. Periodic Properties of the Elements
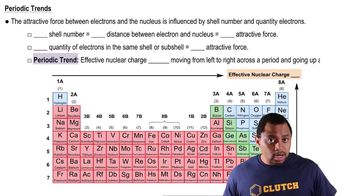
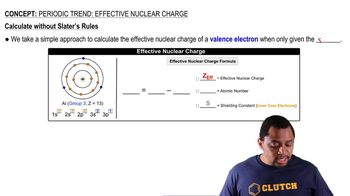
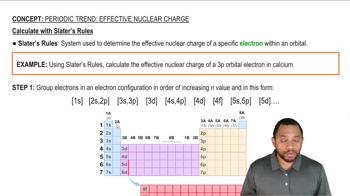
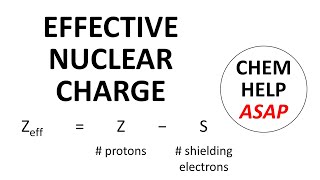
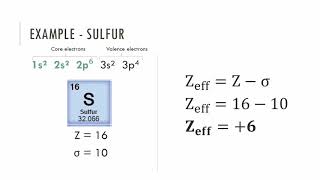
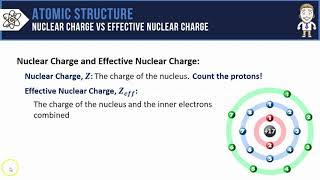
Periodic Trend: Effective Nuclear Charge

Get help from an AI Tutor
Ask a question to get started.
Problem 16
Textbook Question
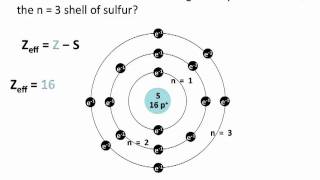
Textbook QuestionDetailed calculations show that the value of Zeff for the outermost electrons in Si and Cl atoms is 4.29+ and 6.12+, respectively. (a) What value do you estimate for Zeff experienced by the outermost electron in both Si and Cl by assuming core electrons contribute 1.00 and valence electrons contribute 0.00 to the screening constant?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
3421
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos