19. Chemical Thermodynamics
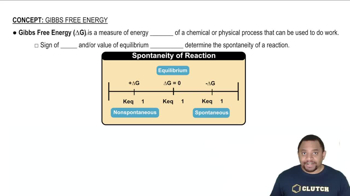
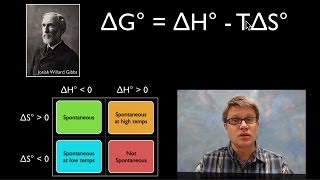
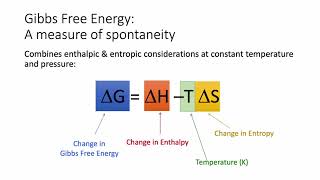
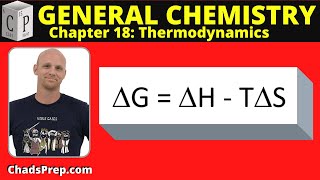
Gibbs Free Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 100c
Textbook Question
Textbook QuestionThe conversion of natural gas, which is mostly methane, into products that contain two or more carbon atoms, such as ethane 1C2H62, is a very important industrial chemical process. In principle, methane can be converted into ethane and hydrogen: 2 CH41g2¡C2H61g2 + H21g2 In practice, this reaction is carried out in the presence of oxygen: 2 CH41g2 + 12O21g2¡C2H61g2 + H2O1g2 (c) Explain how the preceding reactions are an example of driving a nonspontaneous reaction, as discussed in the 'Chemistry and Life' box in Section 19.7.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
557
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos